Natural Solutions for Blue Colors in Food丨Binmei’s phycocyanin
Blue colors are quite rare in nature. Due to the increasing health awareness, the replacement of artificial colorants by their natural counterparts is a major challenge for the food, pharmaceutical, and cosmetics industries. For red, orange, and yellow hues, anthocyanins, betalains, and carotenoids may be used as natural alternatives. In contrast, only a few colorants are commercially available for blue tints. Consequently, how to get natural blue colourants is particularly important. Fortunately, the phycocyanin in spirulina is the perfect solution to all this.
1.1 Origin
1.1.1 Source
Spirulina is the commercial name for phycocyanin, a blue pigment of cyanobacteria and eukaryote algae, such as Rhodophytes and Cryptophytes (Erikson, 2008; Sørensen et al., 2013). The name is due to its main source, the cyanobacterium Arthrospira platensis, commonly named Spirulina platensis. Phycocyanin is an accessory photosynthetic pigment of the phycobiliprotein family. It captures ultraviolet (UV, 200–300 nm) rays and visible light in the yellow to orange spectrum (∼550–700 nm), where chlorophyll and carotenoids exhibit an absorption gap (Chaiklahan et al., 2012). Consequently, the visible color shifts from green to blue.
1.1.2 Cultivation
Phycocyanin is mainly produced by the photoautotrophic cyanobacterium A. platensis, which is cultured in open ponds, raceways, and natural lakes, especially in tropical and subtropical regions (Priyadarshani and Rath, 2012; Sørensen et al., 2013). Despite the generally high contents of phycocyanin in cyanobacteria (60–70 mg/g), productivity is limited because biomass strongly depends on optimal light conditions (Eriksen, 2008). Furthermore, there is a risk of contamination by foreign organisms, causing problems to achieve the hygienic standards required for applications in food and pharmaceutical products. New approaches for cultivation of cyanobacteria, grown photoautotrophically, mixotrophically and heterotrophically, have been comprehensively reviewed by Eriksen (2008). The main prerequisites for commercial pigment production are a rapid growth rate of the microorganisms and low requirements on cultivation conditions; however, accessibility to controlled laboratory culture and ubiquity are important as well (Pandey et al., 2013). Thus, despite the huge variety of cyanobacteria containing phycocyanin, the number of species currently used for commercial production is small.
Eriksen recommended Galdieria sulphuraria, a unicellular rhodophyte evaluated as potential human food source, as an appropriate alternative. Although the pigment content is relatively low (10–25 mg/g), heterotrophic cultivation in the dark enables a large-scale axenic production, resulting in high biomass, and therefore much higher phycocyanin yields. However, due to the cellulose-rich cell wall of algae, causing difficulties in cell disruption and consequently impeding pigment extraction, G. sulphuraria has not yet been used for commercial spirulina production.
1.1.3 Extraction
The process of phycocyanin extraction involves breakage of the cell wall, extraction of the water-soluble protein–pigment complex, followed by concentration, and if necessary, purification. The amount of extractable pigments is significantly determined by the drying process applied to the biomass (Doke, 2005; Oliveira et al., 2008, 2010a; Sarada et al., 1999). A pigment loss of approximately 50% has been reported at elevated temperatures (>50°C), independent of the method (eg, spray drying, oven drying, sun drying). The use of air circulation at ambient temperature without exposure to sun significantly improved extraction efficiency. Optimum pigment recoveries have been achieved through repetitive freeze and thaw cycles using fresh wet materials (Sarada et al., 1999). However, due to limitations regarding storage and transportation, this method is inconvenient and economically unfeasible. Additional problems might be caused by bacterial contamination and the onset of degradation, forming malodorous compounds.
Phycocyanin is extracted by resuspending the dried biomass in water, saline solutions, or sodium phosphate buffer at pH 7. During maceration at temperatures below 30°C, air is passed through the suspension to increase the extraction yield and prevent unpleasant odor (Christiansen et al., 2012). Subsequently, the cell debris is removed by filtration or centrifugation. Further concentration of the crude extract by vacuum distillation at moderate temperatures is required for its use as a food colorant. Nevertheless, regarding food safety, additional sterile filtration appears to be advisable. The addition of sugar improves pigment stability during the heating process (Christiansen et al., 2012). For its application in biomedical research and diagnostics, superior pigment purity is essential. To enhance phycocyanin purity (see Section 2.2.2, Eq. [17.1]) from ∼0.8 in the crude extract to above 4, ammonium sulfate precipitation, ultrafiltration, charcoal adsorption with or without the addition of chitosan, followed by various chromatographic purification steps, have been used (Eriksen, 2008; Patel et al., 2005). However, these methods are time-consuming, involving a large number of processing steps, and are expensive to scale up. Aqueous two-phase extraction using a mixture of polyethylene glycol and potassium phosphates might be an option for phycocyanin purification on a larger scale (Patil et al., 2006, 2008; Rito-Palomares et al., 2001). In addition, the latter method enables fractionation of phycocyanin (C-PC) and allophycocyanin (A-PC) (see Section 2.2.2).
1.2 Chemical Constitution and Related Color and Stability Properties
1.2.1 Structure
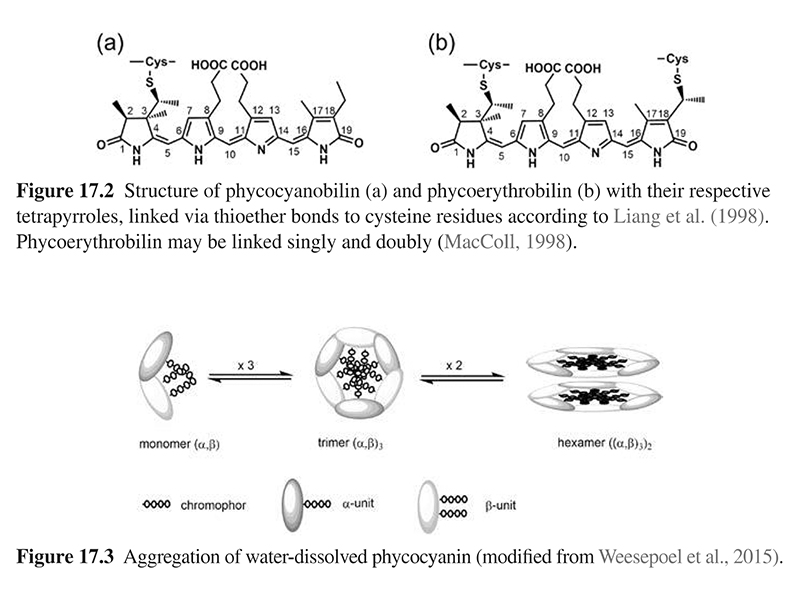
Phycocyanin belongs to the phycobiliproteins, a group of light harvesting apoproteins linked to open-chain tetrapyrroles as prosthetic group (Fig. 17.2) (Chaiklahan et al., 2012; Eriksen, 2008). Phycobiliproteins are classified based on their colors into blue phycocyanin, comprising phycocyanin (C-PC) (Fig. 17.2(a)) and allophycocyanin (A-PC), and red phycoerythrin (Fig. 17.2(b)) with maximum absorbance at 620, 650, and 565 nm, respectively (Kuddus et al., 2013; MacColl, 1998; Pandey et al., 2013). Variations between C-PC and A-PC are caused by different protein structures, modulating the environment of the chromophore, and thereby its optical properties. Saturation of phycoerythrin at C15 shortens the conjugated π-system from eight to six conjugated double bonds, resulting in a strong hypsochromic shift.
Phycocyanins exclusively composed of phycocyanobilin as the chromophore are specified as C-phycocyanins (C-PC). The monomer of C-PC is composed of dissimilar α- and β-polypeptide units (∼20 kDa), containing one and two phycocyanobilins covalently bound to cysteines at position 84 (α) and 84 and 155 (β) (MacColl, 1998; Weesepoel et al., 2015). The monomers aggregate spontaneously to form trimers (α,β)3 and hexamers (α,β)6 (Fig. 17.3). The latter are an integral part of the phycobilisomes—a supramolecular assembly of phycobiliproteins in cyanobacteria and algae. In solution, phycocyanin is a complex mixture of monomers, trimers, hexamers, and other oligomers with molecular weights ranging from 44 to 260 kDa (Chaiklahan et al., 2012; Weesepoel et al., 2015).
1.2.2 Color Properties
Color intensity of commercial spirulina extract depends on pigment concentration and pigment purity. Requirements of the latter vary depending on the respective application. Phycocyanin purity is expressed by UV-visible absorption spectroscopy as the ratio of phycocyanin quantified at 620 nm and colorless proteins assessed at 280 nm (Eq. [17.1]) (Sørensen et al., 2013). However, this method does not distinguish between C- and A-PC, and it involves some imprecision because wavelength at maximum absorption is influenced by the aggregation state, and hence by pH (Jespersen et al., 2005; MacColl, 1998).
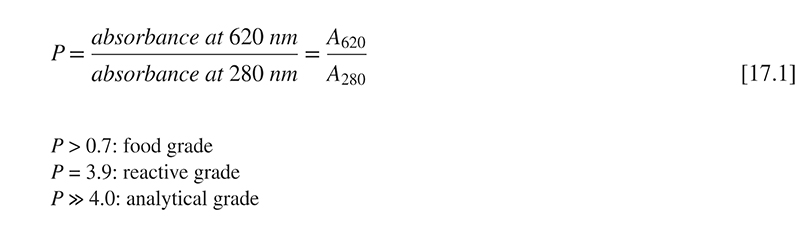
A two-wavelength spectrophotometric method has been developed by Yoshikawa and Belay (2008), to individually determine C-PC and A-PC contents in raw material and complex matrices, such as food and supplements. Phycocyanin is extracted with 100 mM phosphate buffer at pH 6 and incubated for 16 h. After centrifugation, the supernatant is filtered and absorbance read at 620 and 650 nm. Individual concentrations are calculated according to Eqs. [17.2]–[17.5], applying the respective extinction coefficients (Table 17.1).
1.2.3 Stability
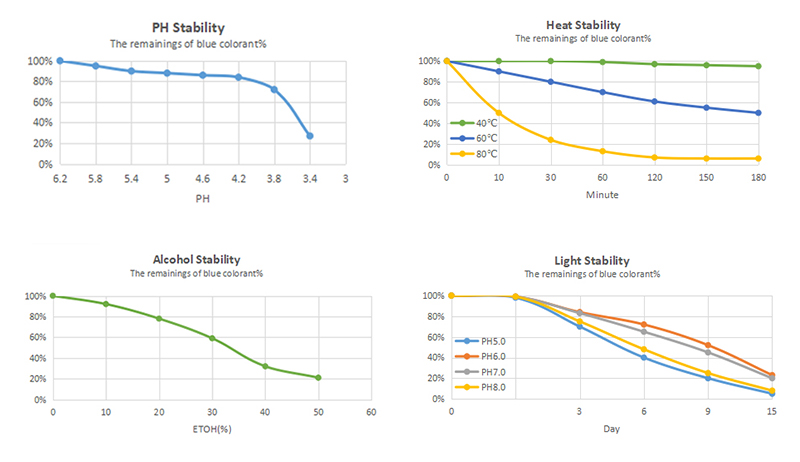
Phycocyanin is soluble in warm and cold water. Around pH 7, its solubility is maximal declining under acidic conditions. Below pH 3, the pigment precipitates immediately due to protein denaturation (Chaiklahan et al., 2012; Mishra et al., 2008). Being a protein, all factors affecting protein structure such as low pH values, high ionic strengths, elevated temperatures, and alcohol concentrations are unfavorable for the pigment. Therefore, control of extraction and purification conditions is mandatory. Depending on pH and pigment concentration, different phycocyanin aggregates are formed (see Section 2.2.2), significantly affecting temperature stability (Chaiklahan et al., 2012; Jespersen et al., 2005).
Phycocyanin is fairly stable at room temperature and under cooled conditions. High color stability has been reported at 0°C and pH 7, with 86% color retention during storage for 45 days (Mishra et al., 2008). In another study, around 80% of the blue color was retained at 4°C (pH 7) over 120 days storage (Chaiklahan et al., 2012). At room temperature, 12% color loss in combination with turbidity and odor has been observed only after 10 days. A further temperature increase to 35°C resulted in almost complete fading within 45 days (Mishra et al., 2008). Beside its quite moderate stability below 45°C, accelerated degradation proceeds at elevated temperatures (Antelo et al., 2008; Chaiklahan et al., 2012; Martelli et al., 2014). Heating at 50, 60, and 70°C for 30 min resulted in 15–30%, 40–50%, and 60–75% color loss, respectively. This high susceptibility to elevated temperature is due to protein denaturation, causing precipitation and fading. Significantly higher color stability has been reported by Patel et al. (2004), with 80–95% color retention during heating over 30 min at 45–65°C (pH 5–7). However, color seems to be barely affected by commonly applied temperature treatment, namely short-time pasteurization (71°C, 15 s, Chaiklahan et al., 2012). In contrast, pasteurization for 1 min at 100°C and 30 min at 80°C was associated with 60% color loss (Martelli et al., 2014).
Some additives are known to enhance phycocyanin stability. Sugar, independent of its type, is the best stabilizer authorized for food use. With increasing concentration, in particular above 40% (w/w), the thermal stability of phycocyanin was drastically improved (Chaiklahan et al., 2012; Martelli et al., 2014). During heating for 1 h at 80°C, 50% of the initial color was retained compared to 7% in the control. Citric acid appeared to be a promising stabilizer as well. The addition of citric acid (4 g/L) to a buffered phycocyanin solution raised color retention from 2.6% (control) to 68% over storage for 45 days at 35°C (Mishra et al., 2008). Also, moderate NaCl concentrations had a positive impact on color stability by attenuating protein denaturation; however, higher concentrations caused precipitation (Chaiklahan et al., 2012).
Phycocyanin is unstable to light. In particular, UV light is deleterious to the dye, and significant bleaching has been reported by Jespersen et al. (2005). Exposure to UV (313 nm) and visible (600 nm) monochromatic light for 1 h, resulted in 9% and 3% (pH 5) and 12% and 2% (pH 7) color loss, respectively. Investigation on biliverdin suggested that methine bridges in the tetrapyrrole chromophore are prone to autoxidation (Weesepoel et al., 2015).
Dufossé et al. (2005) mentioned phycocyanin extracted from the red microalga Porphyridium aerugineum to exert exceptional stability. This pigment did not change color in the pH range of 4–5, and it remained stable following light exposure and thermal treatment (60°C, 40 min).
1.3 Field of Application
Spirulina has been used in Asia for more than 1000 years. Because of its high-quality protein and further valuable ingredients, it has been commercialized as a food supplement (Ali and Saleh, 2012; Borowitzka, 2013; Priyadarshani and Rath, 2012). More recently, spirulina has been considered as a nontoxic, noncarcinogenic natural blue colorant for food and cosmetic applications (Pandey et al., 2013).
In the United States, the FDA has classified spirulina extract as a color additive exempt from certification and approved its use for confectionery (including candy and chewing gum), frostings, ice cream and frozen desserts, dessert coatings and toppings, beverage mixes and powders, yogurts, custards, puddings, cottage cheese, gelatin, breadcrumbs, and ready-to-eat cereals (excluding extruded cereals), at lev- els consistent with good manufacturing practice (21 CFR 73.530). At present, in the European Union, spirulina extract is classified as coloring foodstuff. Consequently, it does not require an E number, thus allowing clean labeling. However, this classification is controversial because it does not consider the selective enrichment of an isolated pigment.
Spirulina is primarily applied to sugar-containing food products with pH values being neutral or slightly acidic. High sugar and protein concentrations and low water content provide optimal conditions for its application. Spirulina is an appropriate dye for sweets, such as chewing gum, coatings, chocolate, sugar decorations, and candies, and for dairy products and ice cream as well. Low temperatures and light exclusion during processing and storage extend the shelf life of the final products. For aqueous food commodities with pH values below 4.5 and alcohol content above 20%, the colorant is far off from industrial applicability (Newsome et al., 2014). Furthermore, spirulina is used as a pigment in cosmetic products such as lipsticks, eyeliners, and eye shadows, but also as an antioxidant, thickening, and water-binding agent, primarily in face, skin, and hair care products and in sun protection (Priyadarshani and Rath, 2012).
Due to its unique fluorescent properties, highly purified phycocyanin is widely used in biomedical research and diagnostics (Chaiklahan et al., 2012; Pandey et al., 2013; Sekar and Chandramohan, 2008). All pycobiliproteins are valuable tracers in several biological assays, such as flow cytometry, fluorescent immunoassays, and fluorescence microscopy.
1.4 Potential Health Benefits
The alga contains up to 60% of well-digestible proteins and a wide spectrum of bio-active compounds, such as polyunsaturated fatty acids, carotenoids, α-tocopherol, vitamins (thiamine, riboflavin, cyanocobalamin), and minerals—chiefly calcium, iron, zinc, magnesium, manganese, and selenium (Borowitzka, 2013; Capelli and Cysewski, 2010; Patel and Goyal, 2013). Spirulina has gained enormous attention from research in recent years. It has been classified as a “superfood,” nutraceutical, and pharmaceutical. Many studies support the assumed benefits from entire spirulina and purified phycocyanin as well. Manifold therapeutic properties such as immune-stimulating effects, antiviral activity (HIV, influenza, herpes), and promising anti-inflammatory, anticancer, and cardioprotective, and neuroprotective activities are discussed (Capelli and Cysewski, 2010; Kamal and Ahmad, 2014; Sekar and Chandramohan, 2008).